
Diabetic Nephropathy Clinical Trial Analysis: Key Insights into Rich Pipeline Featuring 12+ Companies and 15+ Therapies | DelveInsight
The diabetic nephropathy market is driven by the growing burden of diabetes and the increasing demand for effective treatments for kidney disease. Advances in therapeutics, including new drug classes with proven renal benefits, are enhancing patient outcomes and driving market growth. Rising investment in research and heightened awareness of early diagnosis and management are further contributing factors. Additionally, emerging economies with limited treatment access and aging populations offer significant growth potential.
/EIN News/ -- New York, USA, June 10, 2025 (GLOBE NEWSWIRE) -- Diabetic Nephropathy Clinical Trial Analysis: Key Insights into Rich Pipeline Featuring 12+ Companies and 15+ Therapies | DelveInsight
The diabetic nephropathy market is driven by the growing burden of diabetes and the increasing demand for effective treatments for kidney disease. Advances in therapeutics, including new drug classes with proven renal benefits, are enhancing patient outcomes and driving market growth. Rising investment in research and heightened awareness of early diagnosis and management are further contributing factors. Additionally, emerging economies with limited treatment access and aging populations offer significant growth potential.
DelveInsight’s 'Diabetic Nephropathy Pipeline Insight 2025' report provides comprehensive global coverage of pipeline diabetic nephropathy therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the diabetic nephropathy pipeline domain.
Key Takeaways from the Diabetic Nephropathy Pipeline Report
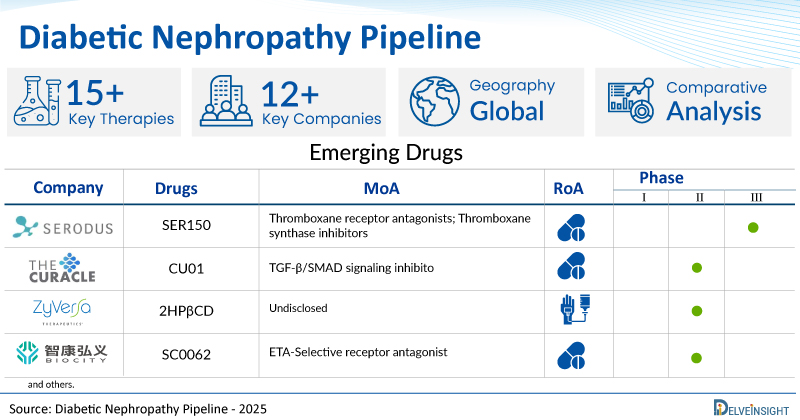
- DelveInsight’s diabetic nephropathy pipeline report depicts a robust space with 12+ active players working to develop 15+ pipeline diabetic nephropathy drugs.
- Key diabetic nephropathy companies such as Serodus AS, Curacle, ZyVersa Therapeutics, Inc., Biocity Biopharmaceutics, Walden Biosciences, Shanghai Alebund Pharmaceuticals, Youngene Therapeutics, Certa Therapeutics, and others are evaluating new diabetic nephropathy drugs to improve the treatment landscape.
- Promising pipeline diabetic nephropathy therapies such as SER150, CU01, 2HPβCD, SC0062, WAL0921, AP 303, YG1805, CTA 382, and others are in different Phases of diabetic nephropathy clinical trials.
- In April 2025, Palatin Technologies announced that data from the Phase IIb BREAKOUT study was presented at the National Kidney Foundation Spring Meeting in Boston, MA. The poster presentation titled Efficacy of Bremelanotide (BMT) to Stabilize Podocyte Function and Reduce Proteinuria in Adults with Diabetic Type II Nephropathy.
- In February 2025, BioCity Biopharma announced that its ETA-Selective receptor antagonist, SC0062, met the 12-week primary endpoint of a reduction in proteinuria in the diabetic kidney disease cohort of its randomized, double-blind, placebo-controlled Phase II trial.
- In September 2024, Walden Biosciences announced that it had completed initial dosing of all subjects in the first cohort of a Phase II basket study evaluating WAL0921 as a treatment for chronic kidney diseases.
- In March 2024, ZyVersa Therapeutics announced Institutional Review Board (IRB) approval of the Phase IIa clinical trial protocol to evaluate the efficacy and safety of Cholesterol Efflux Mediator VAR 200 in patients with diabetic kidney disease.
Request a sample and discover the recent advances in diabetic nephropathy drugs @ Diabetic Nephropathy Pipeline Report
The diabetic nephropathy pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage diabetic nephropathy drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the diabetic nephropathy clinical trial landscape.
Diabetic Nephropathy Overview
Diabetic nephropathy is a serious long-term complication of diabetes that arises when elevated blood sugar levels damage the kidneys’ filtering system. The kidneys are essential for removing waste from the blood, and damage to their filtering units can lead to kidney failure. This condition is a primary contributor to end-stage renal disease (ESRD), which often requires dialysis or a kidney transplant for survival. Diabetic nephropathy usually progresses slowly over several years and may remain asymptomatic until significant kidney damage has already occurred.
Common symptoms of diabetic nephropathy include persistent protein in the urine (proteinuria), swelling in the hands, feet, or ankles due to fluid buildup, frequent nighttime urination (nocturia), fatigue, nausea, vomiting, loss of appetite, unexplained weight loss, and high blood pressure. As kidney function deteriorates further, patients may progress to ESRD. The main cause of this condition is prolonged high blood sugar, which damages the tiny blood vessels in the kidneys. Other contributing factors include genetic predisposition, high blood pressure, smoking, and abnormal blood lipid levels.
Diagnosis involves both urine and blood tests. The urine albumin-to-creatinine ratio (UACR) helps detect early signs of protein leakage, while blood tests measuring serum creatinine and estimated glomerular filtration rate (eGFR) evaluate kidney function. Imaging techniques like ultrasound may also be used to examine the structure of the kidneys and detect any abnormalities. Risk factors for developing diabetic nephropathy include poor blood sugar control, long-term diabetes, high blood pressure, obesity, smoking, and a family history of kidney disease. Certain ethnicities, such as African Americans, Hispanics, and Native Americans, are also at higher risk.
Treatment focuses on slowing kidney damage, managing symptoms, and preventing complications. This involves maintaining optimal blood sugar levels through diet, physical activity, and medications like insulin or oral antidiabetics. Controlling blood pressure is equally important and often includes the use of ACE inhibitors or ARBs, which offer kidney protection. Lifestyle changes such as quitting smoking and maintaining a healthy body weight are crucial. In later stages of the disease, dialysis or a kidney transplant may be necessary. Early detection and effective management of diabetes are key to reducing the impact of diabetic nephropathy and preserving kidney function.
Find out more about diabetic nephropathy drugs @ Diabetic Nephropathy Treatment
A snapshot of the Pipeline Diabetic Nephropathy Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| SER150 | Serodus AS | II/III | Thromboxane receptor antagonists; Thromboxane synthase inhibitors | Oral |
| CU01 | Curacle | II | TGF-β/SMAD signaling inhibitor | Oral |
| 2HPβCD | ZyVersa Therapeutics, Inc. | II | Undisclosed | Intravenous |
| SC0062 | Biocity Biopharmaceutics | II | ETA-Selective receptor antagonist | Oral |
| AP 303 | Shanghai Alebund Pharmaceuticals | I | Undisclosed | Oral |
| CTA 382 | Certa Therapeutics | Preclinical | GPR68 protein antagonists | Undisclosed |
Learn more about the emerging diabetic nephropathy therapies @ Diabetic Nephropathy Clinical Trials
Diabetic Nephropathy Therapeutics Assessment
The diabetic nephropathy pipeline report proffers an integral view of the emerging diabetic nephropathy therapies segmented by stage, product type, molecule type, route of administration, and mechanism of action.
Scope of the Diabetic Nephropathy Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Oral, Intravenous, Subcutaneous, Parenteral, Topical
- Therapeutics Assessment By Molecule Type: Recombinant fusion proteins, Small molecule, Monoclonal antibody, Peptide, Polymer, Gene therapy
- Therapeutics Assessment By Mechanism of Action: Thromboxane receptor antagonists, Thromboxane synthase inhibitors, TGF-β/SMAD signaling inhibitor, ETA-Selective receptor antagonist, GPR68 protein antagonists
- Key Diabetic Nephropathy Companies: Serodus AS, Curacle, ZyVersa Therapeutics, Inc., Biocity Biopharmaceutics, Walden Biosciences, Shanghai Alebund Pharmaceuticals, Youngene Therapeutics, Certa Therapeutics, and others.
- Key Diabetic Nephropathy Pipeline Therapies: SER150, CU01, 2HPβCD, SC0062, WAL0921, AP 303, YG1805, CTA 382, and others.
Dive deep into rich insights for new diabetic nephropathy treatments, visit @ Diabetic Nephropathy Drugs
Table of Contents
| 1. | Diabetic Nephropathy Pipeline Report Introduction |
| 2. | Diabetic Nephropathy Pipeline Report Executive Summary |
| 3. | Diabetic Nephropathy Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Diabetic Nephropathy Clinical Trial Therapeutics |
| 6. | Diabetic Nephropathy Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Diabetic Nephropathy Pipeline: Late-Stage Products (Phase III) |
| 8. | Diabetic Nephropathy Pipeline: Mid-Stage Products (Phase II) |
| 9. | Diabetic Nephropathy Pipeline: Early-Stage Products (Phase I) |
| 10. | Diabetic Nephropathy Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Diabetic Nephropathy Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Diabetic Nephropathy Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the diabetic nephropathy pipeline therapeutics, reach out @ Diabetic Nephropathy Therapeutics
Related Reports
Diabetic Nephropathy Epidemiology Forecast
Diabetic Nephropathy Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted diabetic nephropathy epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Diabetic Nephropathy Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key diabetic nephropathy companies, including Bayer, Janssen Pharmaceutical, AstraZeneca, Daiichi Sankyo, Kyowa Kirin, Teijin America, Boehringer Ingelheim, Eli Lilly and Company, Chinook Therapeutics, Abbvie, Mitsubishi Tanabe Pharma, AstraZeneca, Boehringer Ingelheim, CSL Behring, Gilead Sciences, Goldfinch Bio, Novartis Pharmaceuticals, Prokidney, among others.
Diabetic Peripheral Neuropathy Market
Diabetic Peripheral Neuropathy Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key diabetic peripheral neuropathy companies, including Helixmith, Aptinyx, WinSanTor, Inc., Regenacy Pharmacuticals, Novaremed Ltd., Grünenthal GmbH, Glenmark Pharmaceuticals, among others.
Diabetic Peripheral Neuropathy Pipeline
Diabetic Peripheral Neuropathy Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key diabetic peripheral neuropathy companies, including Apurano Pharmaceuticals, Novaremed, Trevena, Helixmith, Applied Therapeutics, WinSanTor, Centrexion Therapeutics, Praetego, among others.
Type 1 Diabetes Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key type 1 diabetes companies including Landos Biopharma, Diamyd Medical, Gan&Lee Pharmaceuticals, Zealand Pharma, Kamada, AstraZeneca, Novo Nordisk, Provention Bio Preregistration, Histogen, Vertex Pharmaceuticals, Panbela Therapeutics, Arecor, Bioprojet, Novartis, ImCyse, Adocia, Anelixis Therapeutics, Tolerion, TikoMed, Avotres, REMD Biotherapeutics, Novo Nordisk, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn

Contact Us
Shruti Thakur
info@delveinsight.com
+14699457679
www.delveinsight.com
Distribution channels: Banking, Finance & Investment Industry, Healthcare & Pharmaceuticals Industry, Media, Advertising & PR, Science ...
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release

